Veeva Vault PromoMats
Accelerate the End-to-End
Content Lifecycle
Streamline the creation, review, and distribution
of compliant content at scale.
Compliant content at scale
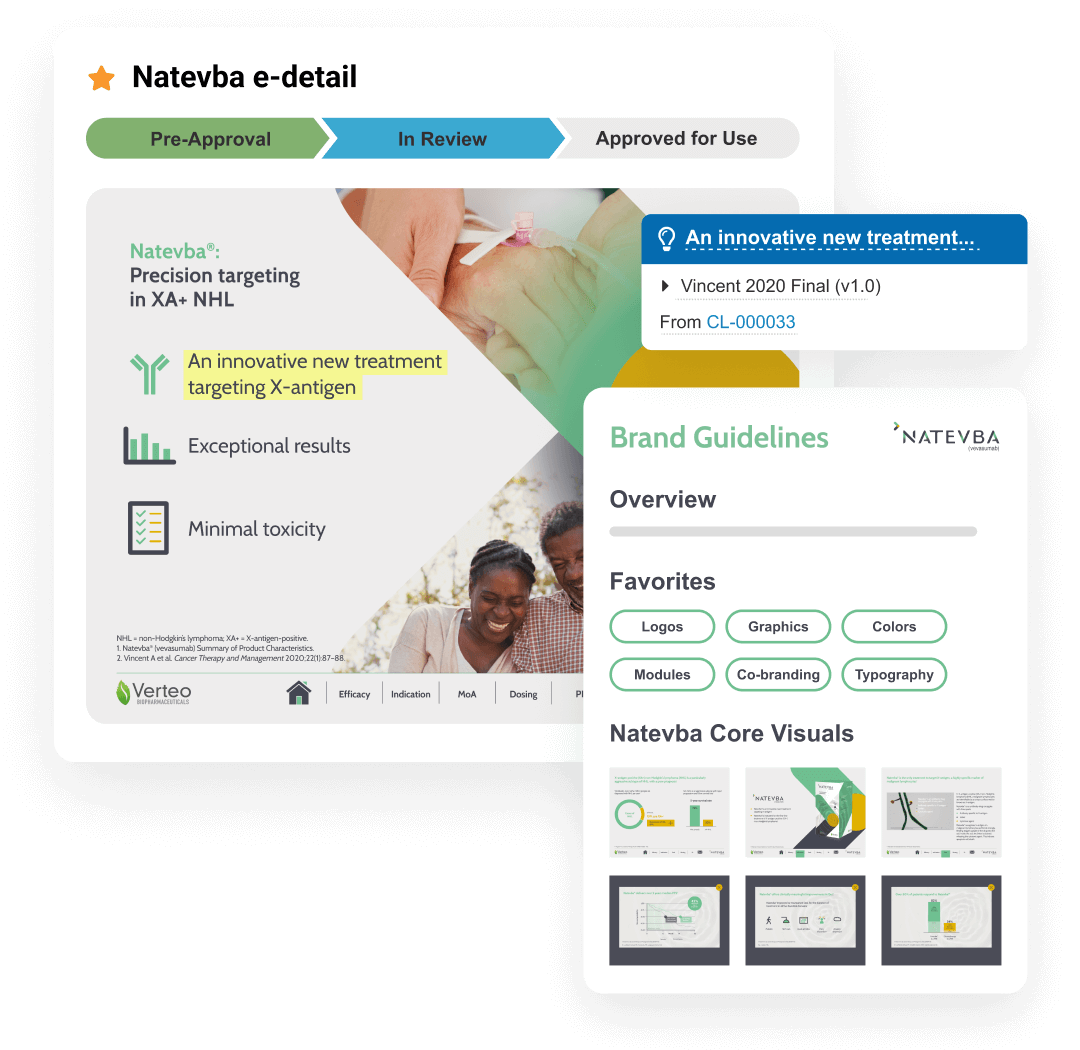
PromoMats is a regulated content management application that supports the full lifecycle of promotional content. It enables content creation, review and approval, digital asset management (DAM), claims management, and modular content.
Connected with CRM for automatic distribution
PromoMats is connected with CRM for automatic distribution of promotional content via applications like CLM and Approved Email. The PromoMats API allows for integration with third-party systems such as web content management or campaign management applications.
Single source of truth for all your content
PromoMats includes Brand Portal, a central repository that makes content easily accessible for internal users while enhancing content reuse. PromoMats generates eCTD compliance packages for digital submission to the FDA.
Why Vault PromoMats
Speed compliant content at scale
Speed content approval
Accelerate content creation and time to market with industry-leading medical, legal, and regulatory (MLR) review.Ensure compliant content across channels
Easily publish and withdraw content to digital channels to make sure only approved assets are in use.Maximize content impact and scale
Scale, reuse assets, and maintain brand consistency with built-in DAM designed for life sciences.
Transform the content lifecycle
+50%
average increase in speed to market
+40%
growth in content reuse with modular content
+20%
reduction in cost to create content




















