VEEVA eClinRO
Simplify eClinRO Management
Provide a simple, web-based application for sites to access,
complete, and manage assessments.
Announced 2023
Status EARLY
Customers 1-10
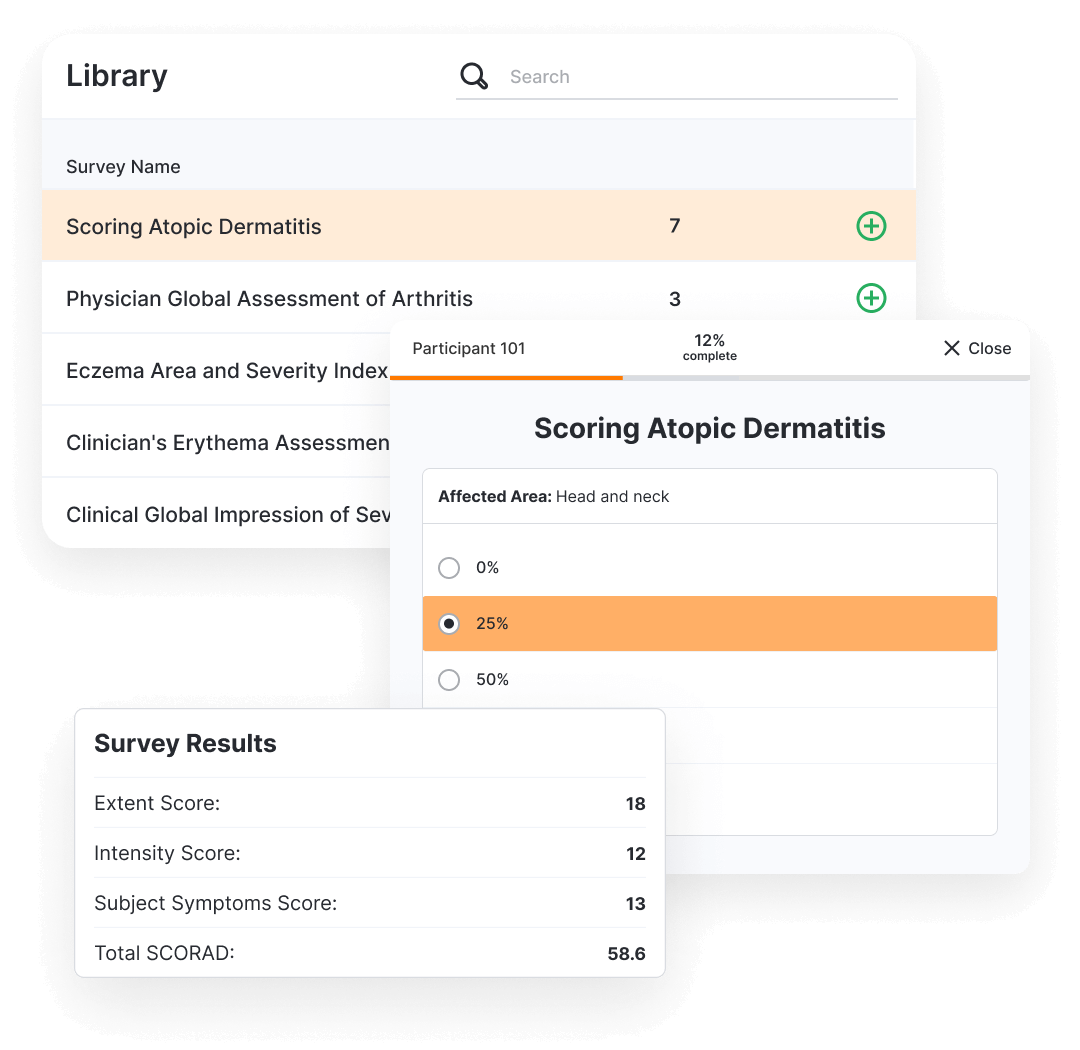
Veeva eClinRO (electronic Clinician Reported Outcomes) captures clinical measurements observed by a trained healthcare professional.
Sponsors manage the eClinROs through their own interface, and a central library allows them to reuse eClinROs across all their studies.
Sites have a single access point to manage their participants, complete eClinROs, and review study progress. They can also review ePRO data and compliance across all their studies within the system.
Once eClinROs are complete, the data flows back into the sponsor’s environment.
Why Veeva eClinRo
Improve site experience
Save time
Manage eClinRO on any web-enabled device with a single login across all studies.Simplify survey collection
Streamline participant setup and eClinRO completion through optimized site workflows.Monitor progress
Enable real-time review of survey status and scoring for sites and CRAs.