Veeva Vault Safety
Global End-to-End Adverse
Event Management
Adverse event intake, processing, submissions,
and oversight with one global modern solution.
Announced 2019
Status EARLY
Customers 51-100
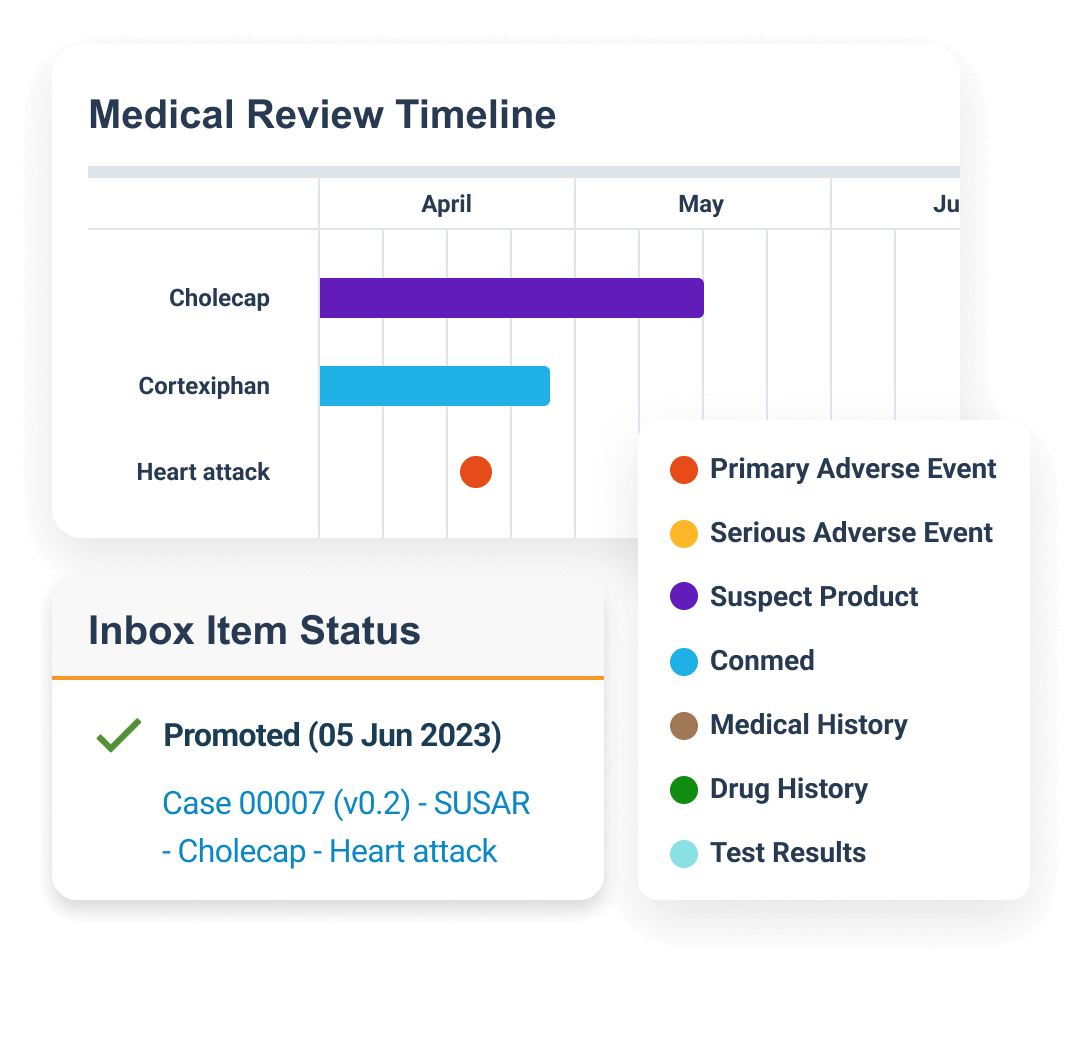
Veeva Vault Safety is a modern safety case management system that manages intake, processing, and submission of adverse events for clinical and marketed products.
Within one system, sponsors and CROs process global and domestic adverse events for drug, biologic, vaccine, device, and combination products. Built-in gateway connections for case submissions and tracking to health authorities and distribution to partners.
Central coding dictionary management automates semi-annual MedDRA, WHODrug, and EDQM updates.
Why Vault Safety
Streamline adverse event management
Improve pharmacovigilance oversight
Real-time reports and dashboards with seamless partner collaboration for risk mitigation and compliance.Streamline intake to submissions
Gain efficiency and automate the end-to-end intake, case processing, and submission process.Global view of adverse events & cases
See all global and domestic adverse events or cases for drug, biologic, device, and combination products.Always stay current
Support new regulatory requirements or access recent capabilities with automatic upgrades.